Nue Fusion Microcurrent
-
 Add to cart
Add to cartOxygen Infusion Skincare & Airbrush Add-On
brightening, A, C, E complex, sensitive, antibacterial & activator
$ 199.00 -
 Select options This product has multiple variants. The options may be chosen on the product page
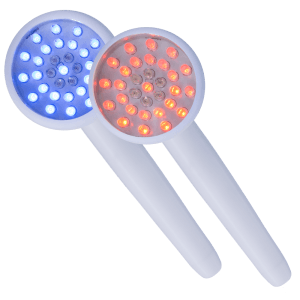
Select options This product has multiple variants. The options may be chosen on the product pageLED Light Therapy
Red & Blue LED Hand Pieces
$ 175.00
More options -
 Select options This product has multiple variants. The options may be chosen on the product page
Select options This product has multiple variants. The options may be chosen on the product pageMicrocurrent Conductivity Gel
odorless & hypoallergenic
$ 19.00
More options -
 Select options This product has multiple variants. The options may be chosen on the product page
Select options This product has multiple variants. The options may be chosen on the product pageMicrocurrent Lead Cord (Wire)
May Include Banana Plugs
$ 10.00
More options -
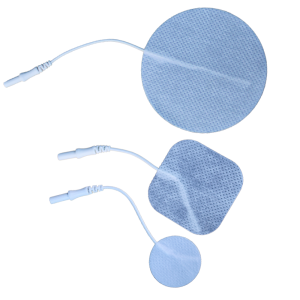 Select options This product has multiple variants. The options may be chosen on the product page
Select options This product has multiple variants. The options may be chosen on the product pageMicrocurrent Neck & Body Microcurrent Pads
Electrode Pads for Nue Fusion Equipment
$ 4.00
More options
Nue Fusion Microcurrent
Nue Fusion Microcurrent is an advanced treatment that may be used for the face and body. Because of its ability to stimulate the mitochondria which are a direct attachment to the myofilaments.
The message pathways increase the contraction of skin and muscle responses for cell-to-cell communication. Building a strong foundation for long-term cumulative result-oriented quality skin health.
Nue Fusion Microcurrent 200 provides an immediate change in skin clarity, firmness, and tightness after one treatment. Creativity is limitless.
Adding various modalities to the face-only machine, such as oxygen infusion, microdermabrasion, dermaplaning, or LED Light therapy is the ultimate in developing a true anti-aging treatment plan approach for long-term quality skin health.
Nue Fusion 600 is ideal for core strength. Whether working with the stomach, waist, or back area. Clients will notice a difference not only in their body image but in their movement. The midline section of the body feels stronger and tighter.
Furthermore, it may also be used on the thighs and buttocks for body contouring. Additionally, we make sure our customers have the right educational materials to reference when working with pad placements.
The Skin for Life microcurrent machine is FDA Registered #1226143. We are the manufacturer of the device. FDA Register means we may sell directly to licensed therapists and physicians. If we were FDA-approved, 510K we could only sell directly to physicians.







